The fatty acids are of two prime types: saturated and unsaturated. A healthy body requires both of them for the optimal functioning of the body. There are certain aspects like their structure, sources, state etc., that make them totally different from each other.
For instance, all the carbon atoms of saturated fatty acids are linked together only with a single bond. In contrast, one or more than one double bonds are present in unsaturated fatty acids.
Nutritionists’ recommendation for consuming saturated and unsaturated fatty acids is around 10% and 30% of our daily calorie intake.
Health professionals that the unsaturated ones are a bit healthier than that of saturated ones. This is because saturated fatty increases good as well as bad cholesterol. But the unsaturated ones increase the level of good cholesterol and decrease the level of bad cholesterol.
The saturated fatty acids are generally solid and don’t get spoiled easily. Whereas unsaturated fats are liquids at room temperature, and they spoil quickly.
In this section, we will know more about the differences between saturated and unsaturated fatty acids.
Content: Saturated Vs Unsaturated Fatty Acids
- Comparison Chart
- Meaning of Fats?
- What are Saturated Fatty Acids?
- What are Unsaturated Fatty Acids?
- Key Differences
- Similarities
- Conclusion
Comparison Chart
| Basis for Comparison | Saturated Fatty Acids | Unsaturated Fatty Acids |
|---|---|---|
| Meaning | Saturated fatty acids contain single chain of carbon atoms with no double bond. | Unsaturated fatty acids contain carbon chains with one or more double bond. |
| Type of Bond | Hydrocarbon chain without double bond (only single bond). | Hydrocarbon chain with one or more double bonds (C=C). |
| Physical appearance | Solid at room temperature. | Liquid at room temperature. |
| Type of chain | Straight chain. | Bend chains at double bond. |
| Melting point | Relatively higher. | Relatively lower. |
| Sources to obtain | Animal fats, palm oil, coconut oil. | Plant and vegetable oil, avocado, sunflower oil, walnuts, flax, canola oil and fish oil. |
| Solubility in vitamins | Soluble in vitamins. | Insoluble in vitamins. |
| Effect of hydrogenation | No effect. | They get converted into saturated state due to hydrogenation. |
| Effect in human | Increased blood cholesterol, deposited in the inner wall of an artery and are harmful to health. | Lowers the blood cholesterol and are associated with health benefits. |
| Shelf life | They do not get spoil quickly and are long-lasting. | They get spoil quickly. |
Meaning of Fats?
As per the findings of Harvard Medical School, fats make a crucial part of a balanced and healthy diet. They significantly contribute to the taste and texture of the food. For instance, the smoothness of guacamole or flakiness of a croissant.
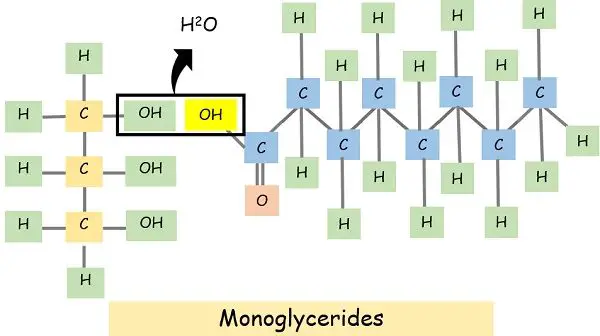
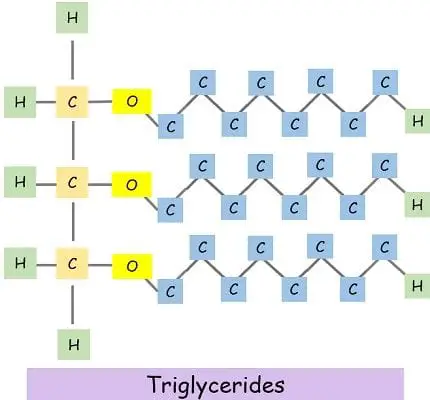
The fats comprise 3 carbon backbones named glycerol and fatty acid chains. When an OH molecule of glycerol binds to the H atom of fatty acid, it releases an H2O molecule.
If the water molecule releases once it generates a monoglyceride. While if it occurs more than once, it is a polyglyceride.
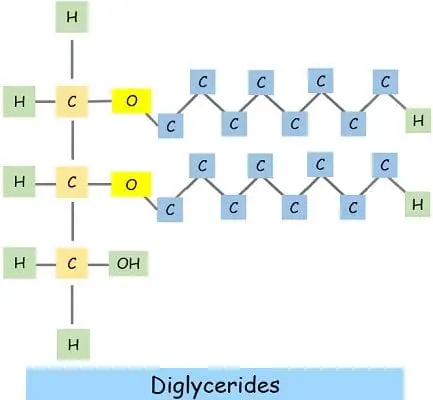
The formation of diglycerides attaches two fatty chains to glycerol with the removal of two water molecules.
Similarly, for the formation of triglyceride, three water molecules move out. And three fatty acid chain link to the glycerol.
Also, they are responsible for executing several metabolic roles in our bodies. Such as:
- Storage the extra energy
- Regulation of body’s temperature
- Insulation of vital organs
- Aiding the clotting mechanism
- To build the membrane of a cell
- To help the absorption of some essential minerals and vitamins
What are Saturated Fatty Acids?
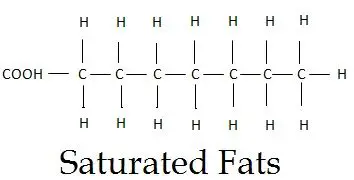
Saturated fatty acids have the simplest structural configurations among other fats. This simplicity is due to the absence of any double bond or branching.
These fatty acids have only single bonds between their carbon atoms. They are completely saturated by the maximum number of hydrogen atoms. Because of this hydrogen saturation and lack of a double bond, there remains no place for any further interaction. Thus, they cannot accept or absorb more of hydrogen atoms.
The triglycerides with saturated bonds are generally straight and linear. Thus, they tend to pack together very well. Due to this compact packaging, they are solids at room temperature.
Definition of Saturated Fatty acids
We can define them as “A linear chain of CH2 molecules combined with a C-C single bond and the carboxylic group at the terminal end”.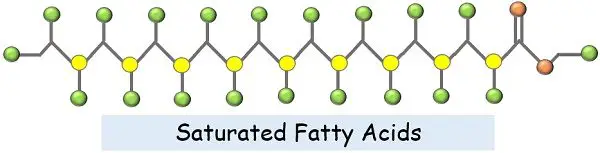
- Capric acid
- Palmitic acid
- Lauric acid
- Myristic acid
- Stearic acid
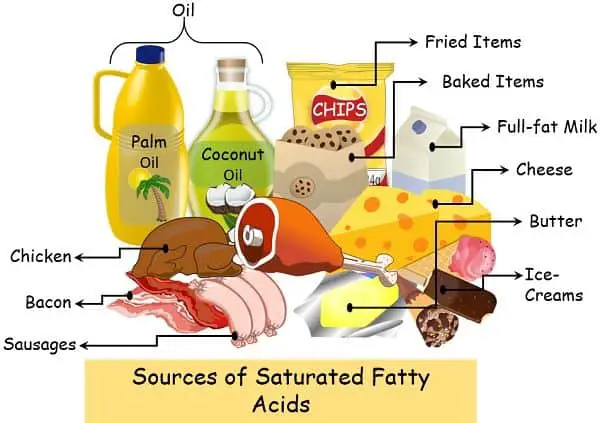
Sources of Saturated Fatty Acids
You can find saturated fatty acids in the food items like:
- Milk and dairy products: This may involve cheese, butter, full-fat milk etc.
- Plants oil: Certain plant oils like palm kernel oil and coconut oil possess higher amounts of these fats.
- Animal meat: This includes beef, pork poultry products.
- Processed meats: Bologna, sausages, bacon and hot dogs are rich sources of saturated fatty acids.
- Other packaged food: Products like chips, cookies, pastries contain saturated fats.
Characteristics of Fatty Acids
- CnH2n + 1COOH is the general formula for saturated fatty acids.
- Mostly these fats are long-chained structures with more than 12 carbon atoms. Butyric acid with 4-C and caproic acid with 6-C are the exceptional cases known as shorter fatty acids.
- The hydrogenation process is not possible with saturated fatty acids. There is no chance of further chemical interaction due to hydrogen saturation.
- Due to the tight packing of the chains, they are solids at room temperature. For this reason, they have a high melting point to melt their solid state.
- Because of their high melting point, their shelf life is more compared to the unsaturated fatty acids.
- They also have lower rancidity levels.
Our Body and Saturated Fatty Acids
Our body needs around 5-10% of the saturated fats in a balanced diet. As per the American Heart Association studies, the amount taken more than this recommended value can severely hamper your cardiovascular system.
They actually are responsible for raising the level of bad cholesterol in our body, which gets deposited in the form of plaque in the arteries.
The bad cholesterol is nothing but LDH, i.e., low-density lipoproteins. This LDH builds up the risk of getting a cardiac disease or type 2 diabetes.
According to the findings of our National Institute of Health, the source of saturation decide the level of risk they impose. For instance, in one of their findings, they found that the saturated fats in dairy products decreased the health hazards. Whereas those which were found in the animal meat enhanced the health risk.
Also, these saturated fats form fat storage beneath the skin by the body due to their solid nature.
What are Unsaturated Fatty Acids?
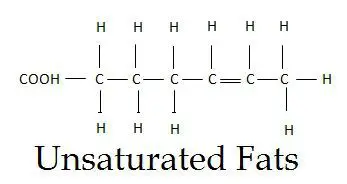
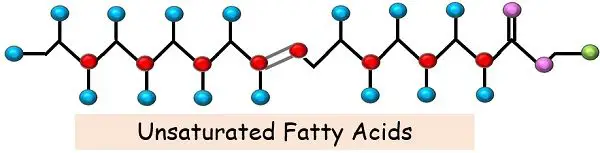
As the name suggests, they are unsaturated or not fully saturated by hydrogen atoms. These are the most complex form of fatty acids due to the presence of the C-C double bond and bending and branching of the fatty acid chain.
In unsaturated fatty acids, the carbons, instead of binding to the maximum number of the hydrogen, they bind to each other, giving rise to a double bond at some places.
There are two fewer hydrogen atoms at the site of every double bond. The presence of the double bond leads to the kink in the fatty acid chain. Because of this kink, they don’t have a linear structure, and thus, they don’t pack smoothly together.
Due to this uneven packaging, unsaturated fats are mainly in the fluid state at room temperature.
Definition of Unsaturated Fatty Acids
We can define unsaturated fats as” An intricate and branched hydrocarbon chain containing one or more C-C double bonds with a carboxylic acid at the terminal end”.
Examples of Unsaturated Fatty Acids
- Linoleic acid
- Linolenic acid
- Crotonic acid
- Oleic acid
- Stearidonic acid
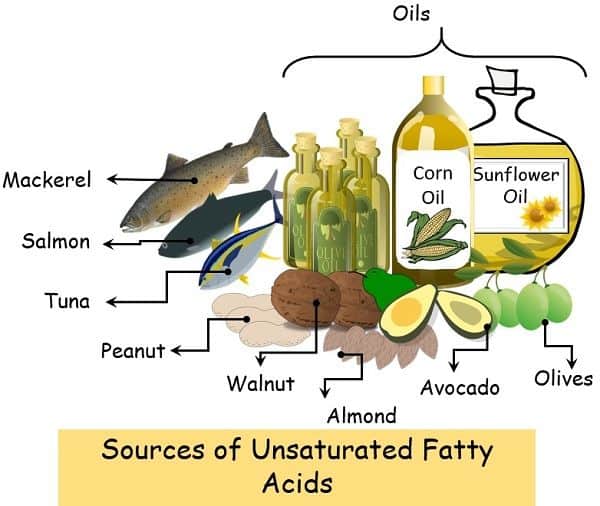
Sources of Unsaturated Fatty Acids
The following is the list of some food items that are a rich source of unsaturated fatty acids:
- Oils: Vegetable oils, sunflower oil, corn oil, canola oil, olive oil etc.
- Nuts: Peanuts, cashews, almonds, walnuts etc.
- Avocado and its products, oil etc.
- Olives
- Fishes: Like salmon, tuna, mackerel, anchovies contain a higher level of omega 3 fatty acids.
Characteristics of Unsaturated Fatty Acids
- The unsaturated word refers to the fact that they are not saturated by the maximum possible number of hydrogen atoms.
- For this reason, they can undergo the process of hydrogenation as they can absorb more hydrogen and convert the double bond into a single bond.
- Their general formula varies with the number of double bonds present in the chain. For example:
- Mono-saturated acids or monoethenoid (One double bond) – CnH2n–1COOH.
- Diethanoids (Two double bonds) – 3COOH.
- Triethnoids (Three double bonds) – CnH2n−5COOH
- Due to the double bond, the fatty acid chain bends a little. Thus, they don’t pack smoothly and are in a liquid state.
- The unsaturated fats have a low melting point. And due to this, their shelf life is relatively low and thus, they spoil quickly.
- These fats have higher chances of oxidation and rancidity.
Types of Unsaturated Fatty Acids
On the basis degree of saturation
The unsaturated fats are of two types based on the number of the double bonds present:
- Monounsaturated Fatty acids (MUFA): These fats contain only a single bond throughout the fatty acid chain.
- Polyunsaturated Fatty acids (PUFA): These fats possess two or more than two double bonds in their structure.
On the basis of the Position of the double bond
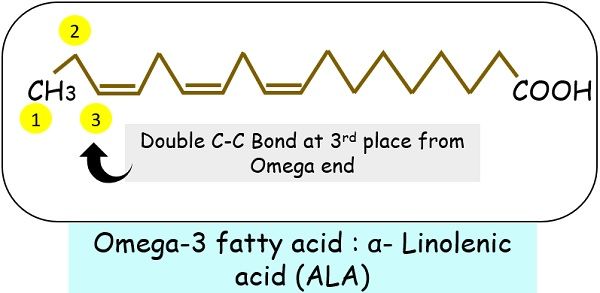
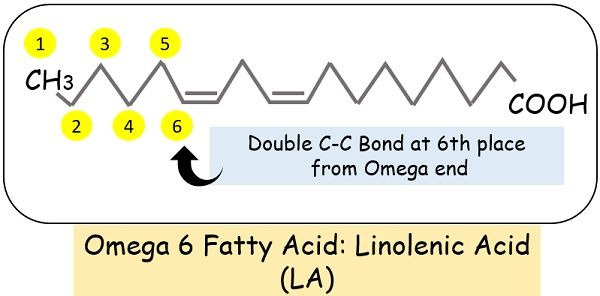
Another name for methyl end is omega. The chain Omega 1,2,3 etc. are based on the location of the double bond.
Some noteworthy examples of omega fatty acids are:
- Omega-3
- Omega-6
- Omega-9
For instance, if the double bond is at the 3rd carbon atom, the fatty acid name will omega-3.
Similarly, if the double bond comes at the 6th place from the Omega end, it is an Omega 6 fatty acid.
On the basis of the conformation of the double bond
According to the orientation of the functional group, there are two categories: Cis and Trans.
- Cis fatty acids: Here, the functional group is present on the same side. During the formation of these fatty acids, the chain naturally bends. When the triglycerides overlap, this bending creates a gap between the chains, making them less compact.
Mostly they are present in the membranes as the gaps between the molecules provide the required fluidity, transport and signalling ability. - Trans fatty acids: In these fatty acids, the functional group attached is on the opposite sides of the chain. Their chains are straight as the functional group are on opposite sides. Also, they pack together very well as there are no gaps between the adjacent molecules.
As these fats are not porous at all, thus they lack the fluidity, ability of transportation, cellular signalling etc.
Our body and Unsaturated fatty acid
Our body requires around 25-30% of the unsaturated fatty acids from our daily diet. These fatty acids are healthier than that of saturated ones.
Unsaturated fats increase the level of good cholesterol in our bodies. Whereas it decreases the level of LDH, i.e., the bad cholesterol, ultimately reducing the risk of cardiac disorders.
The only exception to this is trans fats. They tend to be the unhealthiest fatty acids among all. As they exponentially elevate the LDH value leading to the severe blockage of the arteries. They also burn or lower down the level of good cholesterol already existing in our bodies.
These are dominantly found in junk foods where the cooking oil is used repeatedly. Due to the excessive burning of the oil for several times, the cis fats also get converted into harmful trans fats.
Key Differences Between Saturated and Unsaturated Fatty Acids
- Saturated fatty acids contain a fatty acid chain with a single bond and no branching. Unsaturated fats contain branched carbon chains with one or more C-C double bonds.
- The saturated fatty acids are in the solid state. But the unsaturated fats are in a liquid state at room temperature.
- Due to their solid form, saturated fats have a high melting point. While the unsaturated fatty acids have a low melting point.
- The shelf life of saturated fatty acids is more in comparison to that of unsaturated fatty acids.
- Thus, saturated fatty acids have lower chances of spoilage. Whereas unsaturated fats have higher chances of getting spoiled.
- There is no effect of hydrogenation in saturated fats as the hydrogen atoms fully saturate them. But in unsaturated fats, hydrogenation is possible as the double bonds can break to absorb hydrogens.
- Saturated fats increase the level of bad cholesterol LDH. While unsaturated fats increase the level of good cholesterol in the body.
Similarities
- Both are fats (lipids).
- Hydrogen and oxygen are present in the chains.
- Contains fatty acids and glycerol.
- Both are made of carboxylic acid.
- Act as the energy provider and also help in storing the energy.
- They are insoluble in water.
Conclusion
From the above description and differences, we conclude that not all fats are bad and are meant to obese us. Though we need some specific amount in our daily diet, one should know the goods and the bads of them and their effects on health. As saturated fats increase the level of cholesterol in the blood, also clogs the arteries which raise the chances of attacks or stroke. But unsaturated fats may protect the heart, and its consumption can give a better and healthy life.

Eshetu Girma says
nice note with brief explanation