When the chemical bonds unite any atoms (two or more) is known as a molecule. But such type of molecules that consist of different atoms (two or more) of different chemical elements are known as compounds. However, it is a fact that all compounds are molecules, but all molecules are not compounds.
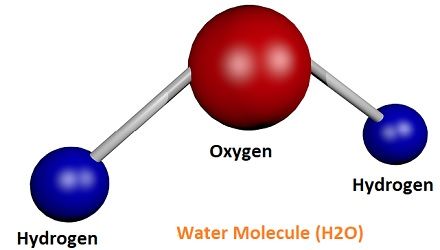
So, we can say that any combination of atoms is a molecule, but when the combination of atoms is from different elements, it is known as the compound. Let’s take an example to understand in a better way, like Hydrogen gas (H2), Oxygen (O2), water (H2O), HCl, Ozone (O3), etc. are the molecules, as they have two or more atoms. On the other hand, Calcium carbonate (CaCO3), NaCl, H2O, etc. are compounds as they are made up of different elements with different atoms.
Secondly, the molecules are determined by the number of atoms taking part in chemical bonding, but compounds are determined by the types of the bond they share between the atoms, which can be either covalent, metallic or ionic bond.
Covalent bonds are such type of chemical bonds, in which atoms share the electrons like in O2 (oxygen molecule). But the ionic or electrovalent bonds are such bonds where the atom donate their electrons to another atom like in NaCl. The bond that is shared between two metal atoms is known as a metallic bond.
Therefore, we can say atoms play the leading role in chemistry, as it is the smallest unit of matter, but when combined with other atoms may give rise to different elements and compounds.
However, there are many things to discuss, but in this article, we will find out the differences between two closely related and confusing terms, which are molecules and compounds.
Content: Molecules Vs Compounds
Comparison Chart
| Basis For Comparison | Molecules | Compounds |
|---|---|---|
| Meaning | When two or more atoms are chemically joined together is known as a molecule. | When two or more different elements are chemically joined together, it is known as the compound. |
| Properties | Molecules can be heteronuclear or homonuclear. | As the compound is in a physical form, they are always stable. |
| Molecules can be unstable. | Compounds are composed of different elements. | |
| Types of Bond | Either has ionic or covalent bonds. | Either has ionic or metallic or covalent bonds. |
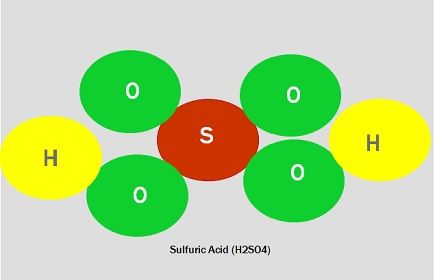
| Examples | Ozone (O3), Oxygen (O2), Dinitrogen (N2), water (H2O), etc. | Calcium carbonate (CaCO3), sodium chloride (NaCl), nitric acid (HNO3), etc. |
Definition of Molecules
When two or more atoms unite to form the recognizable unit, in which the pure substance will be able to retain its chemical properties and composition even after the division of the unit, is known as a molecule.
Molecules may consist of the atoms of the same element, or they can be of different chemical elements. If it contains the atoms from the same chemical element, it is known as homonuclear as in Oxygen (O2) molecule. But if the molecule is composed of different atoms and elements, it is known as heteronuclear like water (H2O).
Types of Molecules
Diatomic, Triatomic, Polyatomic molecules: Based on the number of atoms per molecule.
Homonuclear, Heteronuclear, Molecules: Such molecules that contain atoms of the same element is homonuclear, while molecules with atoms of different chemical elements.
Organic, Inorganic Molecules: Such molecules that composed C, H elements, with the addition of other elements are organic molecules, whereas molecules with different combinations of elements are inorganic molecules.
Covalent, Ionic Molecule: Molecules based on the bond they share which can be either covalent or ionic.
Examples: HCl, CO2, H3+,NaCl, NO2, P4, PCl5, C6H12O6, etc.
Definition of Compounds
A group of different atoms and different elements united together by the chemical bond is known as the compound. The chemical bond can be covalent or ionic or metallic.
As said earlier, all compounds are molecules, but not all molecules are compounds.
Types of compounds
Diatomic, Triatomic, Polyatomic: Such compounds that vary on the basis of the number of atoms present.
Simple and Complex Compounds: Compounds are differentiated on the basis of complexity.
Organic and Inorganic Compounds: On the basis of components, like carboxylic acids, amines, alcohols, hydrocarbons, amides, etc., falls under the organic compound. Nitrites, nitrates, hydrides, oxides, carbonates, halides, etc. comes under inorganic compounds.
Covalent and Ionic Compounds: Differentiation is on the basis of covalent bonds or ionic bond.
An important point to consider is that only heteronuclear molecules are categorized as compounds, but not homonuclear molecules.
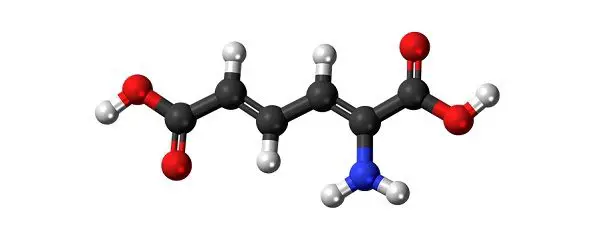
Examples: Acetic acid (C2H4O2), Alcohol (C2H6O), Ammonia (NH3), Hydrogen Peroxide (H2O2), H2O, NaCl, etc.
Compounds are presented by their chemical formula. Like, H2O is the chemical formula for water, which shows that the compound has two molecules of hydrogen with one molecule of oxygen.
Key Differences Between Molecules and Compounds
Following are the critical to identify the differences between the molecules and compound:
- When two or more atoms are chemically joined together is known as a molecule. On the other hand, when two or more different elements with different atoms are chemically joined together, it is known as the compound.
- Molecules can be heteronuclear or homonuclear and are unstable; however, Compounds are heteronuclear molecules and are always stable as they are in physical form.
- Molecules may have either ionic or covalent bonds, whereas compounds have either ionic or metallic or covalent bonds.
- Ozone (O3), Oxygen (O2), Dinitrogen (N2), water (H2O), etc., are examples of molecules, whereas Calcium carbonate (CaCO3), sodium chloride (NaCl), nitric acid (HNO3), etc., are an example of compounds.
Conclusion
Molecules and compounds are the most used terms in the field of chemistry. This article was presented to discuss the essential differences between the two words, i.e. molecules and compounds in the comparison chart and also with the help of critical differences. This may help the reader to understand the concept more clearly.

Leave a Reply