Both gas and liquid chromatography serve the same purpose, i.e., separating the components of any mixture. But they vary greatly if we talk about their working mechanisms, components used and the required conditions.
Gas chromatography uses gas as its mobile phase for moving the particles of the sample inside the column. In contrast, in liquid chromatography, the mobile phase used to move sample particles is liquid in nature.
The principle behind gas chromatography is that it uses, separates and analyses the compounds in their volatile or gaseous state. In contrast, the liquid chromatography technique separates the dissolved ions, particles or molecules in a liquid.
The gas chromatography works under a very high temperature to keep the sample in its volatile state. In comparison, liquid chromatography works under high-pressure conditions that can push and separate the constituent particles of a sample.
In analytical chemistry, people often get confused between these two important chromatographic techniques. Thus, here we aim to provide you with all the major differences between gas and liquid chromatography.
Content: Gas Vs Liquid Chromatography
- Comparison Chart
- What is Gas Chromatography?
- What is Liquid Chromatography?
- Key Differences
- Conclusion
Comparison Chart
| Basis of Characteristics | Gas Chromatography | Liquid Chromatography |
|---|---|---|
| Meaning | It is the chromatographic technique that aids the separation and analysis of volatile compounds in a gaseous phase. | It is the chromatographic technique which separates ions or molecules present in the dissolved state in a solvent. |
| Also Known as | Gas-liquid chromatography or vapour-based chromatography | Liquid-solid chromatography |
| Mobile phase | Gas Example: Helium | Liquid Example: Silica |
| Stationary phase | Liquid molecules present in the solid support | Solid adsorbent |
| Sample | Volatile | Comparatively less volatile |
| Chromatographic Bed Shape | Column | Column or plane |
| Detectors | The quality of resolutions depends on the volatility of the components present in the mixture | Here the detectors are: • Ultraviolet-visible (UV-Vis) spectroscopic detector • Refractive Index detector (RID) • LC/MS |
| Column | Thin, long and narrow capillaries or packed columns | Short and wide-sized packed columns |
| Dependency of Resolution | The quality of resolutions depends on the volatility of the components present in the mixture | Here, the resolution relies on the composition of the mixture and the polarity of the molecules present. |
| Operative Conditions | Takes place in high temperatures | Works under high pressure |
| Temperature Control | Required | Not-required |
| Use | In the separation of the volatile compounds | In the separation of the soluble constituent molecules |
| Expense | Low-cost | High-cost |
| Time consumption | Takes comparatively less time | Very time -a consuming process |
| Application | • Separation of oils and fatty acids • Separating the plant’s pigment • Drug abuse testing • Isolating the volatile pesticides from the sample • Testing the toxins and other harmful chemicals in the air | • Separation of inorganic ions from the sample • Separation of sugars, polymers, nucleotides, vitamins, peptides, proteins, lipid molecules etc. |
What is Gas Chromatography?
Gas chromatography is a very renowned technique in analytical chemistry used to separate and analyse the constituents of the compounds in their volatile state.
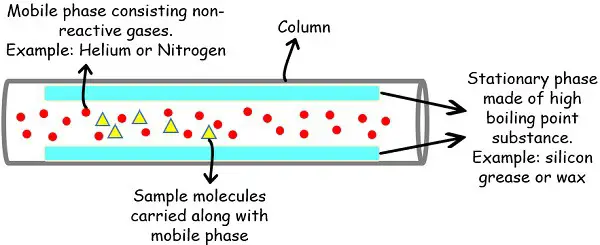
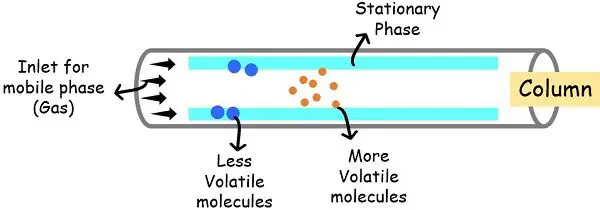
Gas chromatography uses gas as a mobile phase to carry the sample molecules over the stationary phase. This gaseous phase carries the sample molecules on the stationary phase inside the column.
Separation of Components
The separation of the components in gas chromatography relies on the partition equilibrium of the components between the mobile and stationary phase.
The level of separation depends upon the boiling point of the components. The higher the boiling point, the more slowly the component moves through the stationary phase.
The molecules with a low boiling point interact less with the stationary phase and more with the mobile phase, moving faster through the column.
Whereas those with higher boiling points interact less with the mobile phase and more with the stationary phase, moving slowly within the column.
Components of Gas Chromatography
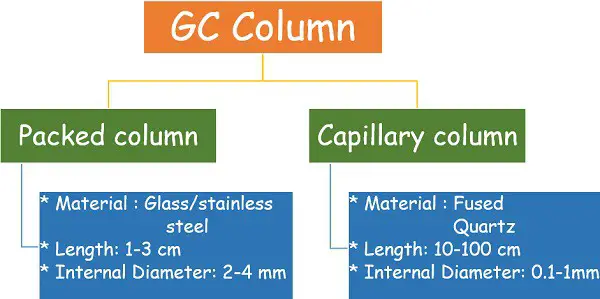
Column
It is a long-tube-like coil that remains compactly arranged in a chamber. The column contains an adsorbent material that acts as the stationary phase for the chromatography.
- Packed column
- Capillary column
Sample
The sample should be volatile if you want to analyse it with gas chromatography. It can be either in gaseous or liquid form.
If it is in the liquid state, the sample needs to get converted into its vaporised form.
Stationary Phase
The stationary phase provides the platform on which the molecules run. The inner walls of the column have the lining of adsorbent material that acts as a stationary phase.
Both liquids, as well as solid materials, can serve the role of the stationary phase. Moreover, the material used must be able to bear very high temperatures in the chamber or oven.
Mobile Phase
The gas used for the mobile phase is generally inert or unreactive by nature. This avoids any chemical activity between sample and gas or gas and stationary phase.
Mostly the analytical laboratories prefer helium or nitrogen gas for this purpose since the former is inert while the latter is unreactive.
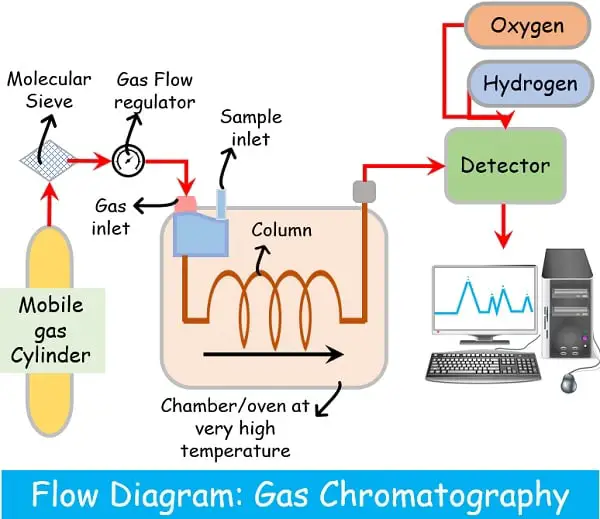
Molecular sieve
This remain attached between the gas cylinder and the chamber. It filters out all the unwanted contaminations like hydrocarbons, oxygen or water content that could hamper the process.
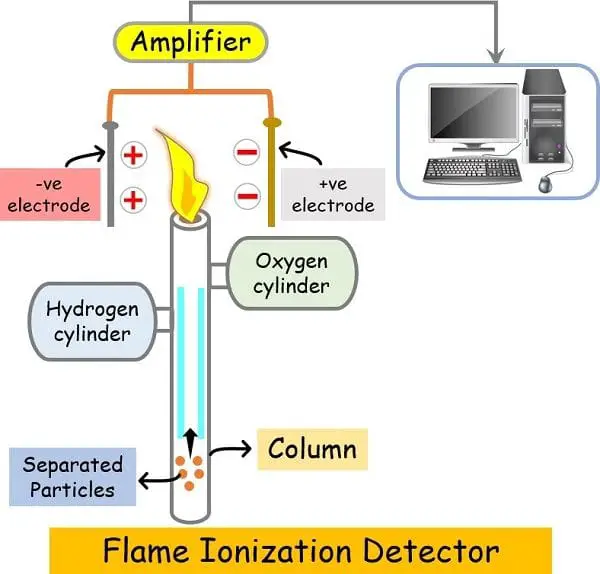
Detector
A detector detects the elute molecules coming out of the column at the end of the column.
There are several types of detectors present in gas chromatography like:
- Flame ionisation detector (FID)
- Thermal conductivity detector (TCD)
- GS/MS
Working on Gas Chromatography
The sample to be separated is prepared by mixing with an appropriate volatile solvent such as heptane, acetone or methanol. The sample is injected into the injecting port.
The injection port temperature remains 20-50 degrees higher than the column. This allows the rapid volatilisation of the sample.
The mobile gas is then released into the column, and the sample starts.
The constituents separate according to their respective boiling points.
Detection of results in Gas chromatography
As soon as the samples run via column, the detector detects the elute. After it sends the signals to the computer to generate a peak.
They give a peak concerning the retention time of the sample.
The area under the peak gives information about the concentration of the sample.
Understanding Gas Chromatography with example
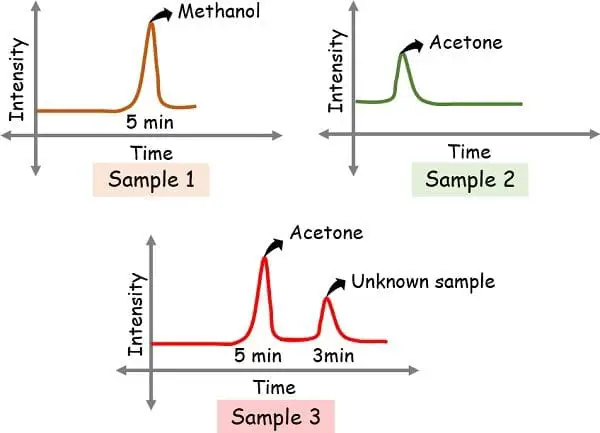
Suppose if we run three samples,
- Containing methanol: Sample 1
- Containing acetone: Sample 2
- Unknown: Sample 3
The peak in methanol came at 5 min while that of acetone came at 3 min.
So, when the unknown sample shows the peak at 5 min and 8 min, then it makes it confirms that the one obtained at 5 min is methanol. And, since the graph indicates no peak at 3 min, this indicates the absence of acetone.
What is Liquid Chromatography?
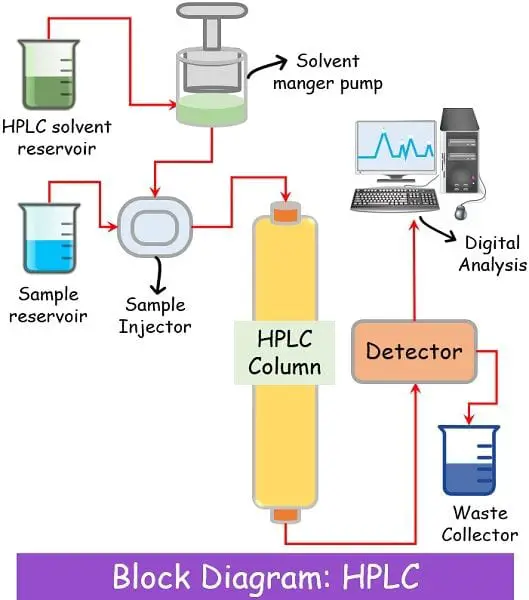
Liquid chromatography is the most advanced type of chromatography which uses liquid as its mobile phase. Currently, liquid chromatography refers to high-performance liquid chromatography (HPLC).
As the name suggests, high performance means high resolution, while liquid chromatography indicates the liquid mobile phase. Moreover, HPLC is referred to as high-pressure liquid chromatography in some places since it works under very high pressure.
Principle Behind Liquid Chromatography
The working principle of Liquid chromatography or HPLC is that of others. The principle starts with equilibration, where the column is equilibrated with buffer followed by sample binding, wash, and ultimately elution.
Types of HPLC
There are two primary types of HPLC based on the polarity of stationary and mobile phases:
- Normal phase: The mobile phase is non-polar while stationary is polar.
- Reverse phase: The mobile phase is polar, and the stationary phase is non-polar.
Components of Liquid Chromatography
Column
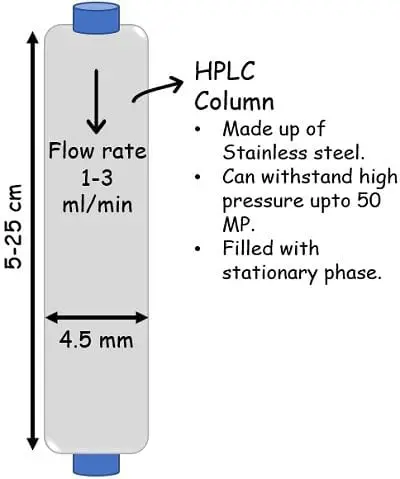
The column is tightly packed with small-sized (2 to 50 microns) adsorbing material. The columns have such a design that they can work efficiently under high-pressure conditions that can range from 50-350 bar. The material used for building the columns is quite sturdy so as to bear the high pressure.
- Affinity column
- Gel filtration column
- Ion exchange column
Stationary Phase
The HPLC column includes adsorbent materials like silica, hydroxyapatite media, or polystyrene resins/beads. Example: Polystyrene divinylbenzene.
Beads with smaller diameter results in improved separation with increased resolution. This is because it provides larger surface areas for the sample molecules to interact.
Mobile Phase
This technique uses a mixture of different solvents as a mobile phase.
The selection of the mobile phase depends on the type of sample you are separating.
There is a separate mobile reservoir to store the mobile phase liquid. This reservoir remains attached to a pump that pushes the mobile into the column with high pressure.
Detector
Like gas chromatography, different types of detectors remain attached at the end of the column for an analysis of the elute coming out.
Some common detectors are:
- IR detector
- UV detector
- Fluorescence detector
- Mass spectrometer
- Refractive index detection
- Electrochemical detectors
Understanding Liquid Chromatography with Example
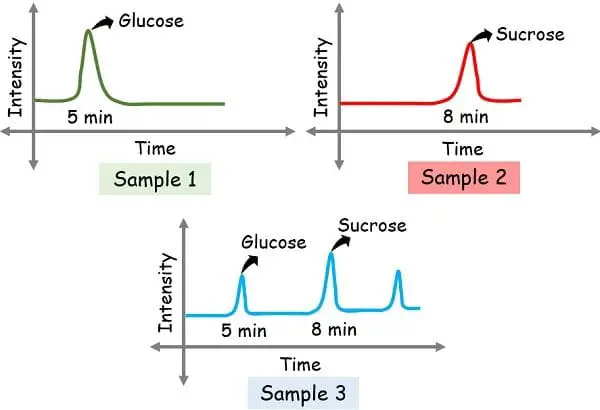
In order to detect the constituents of the sample, you need a standard reference to compare. Thus, the known samples are run for reference.
For example, we had run samples 1 glucose and 2 sucrose as known references and obtained the peak at 5 min and 8 min. And then we had run sample 3 as unknown.
After running the unknown the peaks obtained at 5 min and 8 mins confirm the presence of glucose and sucrose respectively.
Key Differences Between Gas and Liquid Chromatography
- The gas chromatography separates and analyses compounds in their volatile phase. In contrast, liquid chromatography is useful for partitioning constituent molecules in a soluble state.
- The mobile phase is gas in the case of gas chromatography. In comparison, the mobile phase is liquid in liquid chromatography.
- The stationary phase can be liquid or gas in gas chromatography. In comparison, that is solid adsorbing material in liquid chromatography.
- The column is long and narrow, packed or capillary in gas chromatography. While the column is short and wide; packed columns.
- Gas chromatography works under high temperature, whereas liquid chromatography works under high pressure.
- The gas chromatography technique is a comparatively low-cost affair. But the liquid chromatography is highly expensive.
Conclusion
The post will provide the key differences between gas and liquid chromatography along with a detailed overview of their components, working mechanisms and detection.

Leave a Reply