The two types of nitrogenous bases ‘Purines and Pyrimidines‘ differ in their structure. Purine is composed of an organic ring having two nitrogen and four carbon atoms fused with an imidazole ring, whereas pyrimidine structure is similar to that of benzene having two nitrogen and four carbon atoms only.
Purines and Pyrimidines are very known as the backbone for the formation of DNA and RNA. These are the aromatic heterocyclic compounds, present in cells and are involved in protein synthesis, cell signalling, energy storage, mechanisms of enzymes.
As these are the aromatic structures that have carbon and nitrogen in their rings (heterocyclic). Purines and pyrimidines structure are similar to pyridine and benzene, where the exception is of the nitrogen atom.
Guanine and adenine are the two types of purines, and thymine, uracil and cytosine are the three types of pyrimidines are present in the nucleic acid structure.
If we discuss the structure of the nucleotides, they are composed up of a pentose sugar, nitrogenous bases (purines and pyrimidines) and a phosphate. Nucleotides are the monomeric units of the nucleic acids, or we can say that nucleic acid is made up of nucleotides by the bridge of 3′ and 5′ phosphate bonds.
In the formation of the DNA strand, each purine (two rings) of one strand pairs up with the pyrimidine (one ring) of the corresponding strand with the help of hydrogen bonds or vice versa. This is known as a base pairing, and this can be separated at the time of replication or transcription. As purines always form hydrogen bonds with a pyrimidine, it is known as complementary base pairing.
So as per the Chargaff’s rule, the pairing in DNA is – A::T and G::C. But in case of RNA, the pyrimidine thymine is replaced by uracil, so pairing is between – A::U and G::C.
While, there are many points to discuss in many respects on these terms, concerning their similarities and differences. However, our priority is to understand the points on which the purines and pyrimidines differ. We will also go through a brief description of them.
Content: Purines Vs Pyrimidines
Comparison Chart
| Basis for Comparison | Purines | Pyrimidines |
|---|---|---|
| Meaning | A heterocyclic aromatic organic compound, composed of a pyrimidine ring fused with an imidazole ring. | Pyrimidine is also a heterocyclic aromatic compound, and the structure is similar to the rings of benzene and pyridine, composed of carbon and nitrogen. |
| Molecular Formula | C5H4N4. | C4H4N2. |
| Nitrogenous Bases | Guanine, and Adenine in both DNA and RNA. | Uracil in RNA only; Cytosine in DNA as well as in RNA and Thymine in DNA only. |
| Molar Mass | 120.115 g.mol-1. | 80.088 g mol-1. |
| Structure | Double ring (two carbon-nitrogen rings with four nitrogen atoms). | Single ring (one carbon-nitrogen ring with two nitrogen atoms). |
| Melting point | Purine has a melting point as 214-degree Celsius (417 degrees Fahrenheit). | Pyrimidine has a melting point as 20-22 degree Celsius (68 to 72 degree Fahrenheit). |
| Catabolism | Uric acid is produced after the catabolism. | Ammonia, carbon dioxide and beta-amino acids are produced after catabolism. |
| Uses | They are naturally present in our body and forms the chain of DNA and RNA. They are used as drugs, proteins and in enzyme regulation. | Pyrimidines are also present in our body and form the chain of DNA and RNA. They are used in starch synthesis, protein, energy storage, drugs. |
Definition of Purines
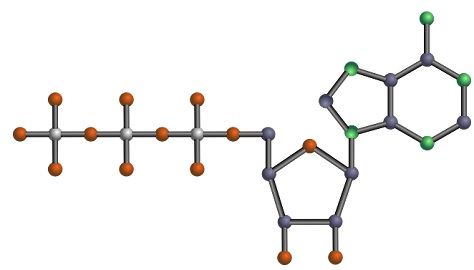
Purines are heterocyclic aromatic organic compound, composed of a pyrimidine ring fused with an imidazole ring. The type of chemical compound, present in various foods, especially in meat products, seafood alcoholic beverages like beer. Purines are water-soluble. The structure of purine is the fusion of the pyrimidine ring with an imidazole ring, and so it is bigger than the pyrimidine.
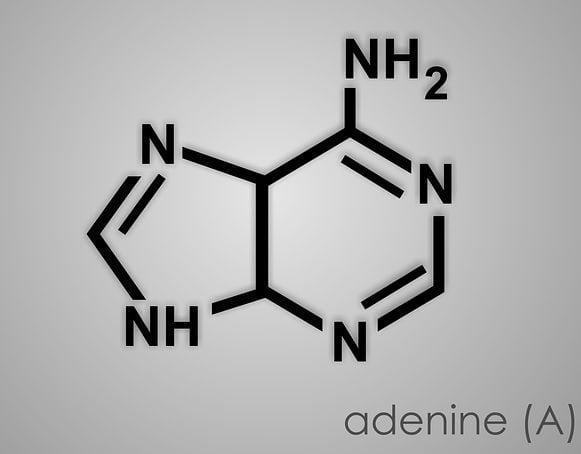
Purines are a matter of study, as they are the most vital part of plant and animal cells which DNA and RNA. The name ‘purines’ is given to the compounds containing nitrogen and carbon atoms. The two known purines found in DNA and RNA are adenine and guanine. While others are caffeine, uric acid, xanthine, hypoxanthine, isoguanine and theobromine. Purines also participate in the formation of biomolecules like GTP, cyclic AMP, NAD, ATP, and coenzyme A.
Purines are categorized into two parts in the human body; Endogenous purines and Exogenous purines. The body itself produces endogenous purines.
Exogenous purines are taken externally by the food we eat. These are metabolized by the body and produce a waste product known as uric acid, which is excreted through urine.
If the uric acid produced in high amount in the bloodstream, there occurs a condition known as hyperuricemia. This may also lead to kidney stones or gout (inflammatory joint condition). So person suffering from hyperuricemia or gout, are advised to consume less amount of such food that has a high quantity of purines.
Definition of Pyrimidines
Pyrimidine, having the molecular formula as C4H4N2, is the simplest member of the family of organic compounds characterized by heterocyclic series with the ring structure. The ring structure has two nitrogen and four carbons.
Likewise, the purines, pyrimidines are also present in the DNA and RNA. Three of the well-known pyrimidines are uracil, thymine and cytosine. Among them, thymine and cytosine are found in the present as the nucleobases in DNA, while uracil and cytosine in RNA. To form the complementary base pairing, purines forms hydrogen bonds with pyrimidines.
The number of bonds also vary while base pairing, as cytosine, always base pairs with guanine and forms three hydrogen bonds in DNA and RNA. But adenine while pairing with the thymine always forms two hydrogen bonds in DNA and RNA, adenine pairs with two hydrogen bonds to uracil.
Key Differences Between Purines and Pyrimidines
Following are few though important point that highlights the differences between purines and pyrimidines:
- Purine is a heterocyclic aromatic organic compound, composed of a pyrimidine ring fused with an imidazole ring, on the other hand, pyrimidine is also heterocyclic aromatic compound, the structure is similar to the rings of benzene and pyridine, composed of carbon and nitrogen.
- Molecular Formula of purine is C5H4N4, while pyrimidine has C4H4N2.
- Guanine and Adenine are the purines present in both DNA and RNA; in case of pyrimidines Uracil in RNA only; Cytosine in DNA as well as in RNA and Thymine in DNA only.
- Molar Mass of purine is 120.115 g.mol-1 and pyrimidine has 80.088 g mol-1.
- Purine has a double ring (two carbon-nitrogen rings with four nitrogen atoms), while pyrimidine has a single ring (one carbon-nitrogen ring with two nitrogen atoms).
- The melting point of purine 214-degree Celsius (417 degrees Fahrenheit), while pyrimidine has a melting point as 20-22 degree Celsius (68 to 72 degree Fahrenheit).
- Uric acid is produced after the catabolism of purines, on the other hand, ammonia, carbon dioxide and beta-amino acids are produced after catabolism of pyrimidines.
- Purines are naturally present in our body and form the chain of DNA and RNA, and these are also used as drugs, proteins and in enzyme regulation. Pyrimidines are also present in our body and form the chain of DNA and RNA; these are also used in starch synthesis, like protein, energy storage, drugs.
Conclusion
In biochemistry purines and pyrimidines, are given a special place as they are building blocks of the DNA and RNA, and are the storage of genetic information. In this article, we came to know about these compounds, the points on which they differ and the way they form the hydrogen bonds while base pairing. Purines are also the energy sources, while pyrimidines are used in many therapeutic dose and antibiotics.


Leave a Reply