Niels Bohr postulates the atomic model which states that electrons move in specific circular orbits around the nucleus with quantized kinetic and potential energies. Ernest Rutherford said that an atom consists of the whole mass that is concentrated into the center.
From the above statement, we can say that both the models of the atom differ in the way they explained the movements of electrons around the nucleus and their energy levels. We all are aware of the modern theory which says that “matter is made up of tiny particles known as atoms which in turn are made up of subatomic particles”.
The Atomic theory is the scientific explanation of the properties of atoms and matter that combines factors of physics, chemistry, and mathematics. The word ‘atoms’ is derived from the Greek word ‘atomos‘, meaning ‘indivisible‘. Though Atomic theory is the concept that originated in ancient India and Greece.
So, it can be said that the Atomic theory has crossed the thousands of years, when it begins in the 5th century B.C., with Democritus theory “matter consists of the indivisible, indestructible units that interact with each other“. Moving onto the 18th century when Dalton’s proposed the atomic theory and then passing in the 20th century with the discovery of subatomic particles, the plum pudding model, Rutherford model, Bohr model, and quantum theory, the journey was full of discoveries and information.
But the two milestones along the whole path is the Bohr atomic theory, which was the modified version of the Rutherford atomic model and so sometimes named as Rutherford-Bohr atomic model. With reference to these theories, in this article, we will be highlighting the difference between the two, with a brief explanation of their work.
Content: Bohr Vs Rutherford’s Atomic Models
Comparison Chart
| Basis for Comparison | Bohr Atomic Model | Rutherford Atomic Model |
|---|---|---|
| Meaning | Rutherford theory says that in the centre of an atom is a small positively charged nucleus, which is surrounded by the negatively charged electrons. | The Bohr atomic theory depicts that atom which as a positively charged, small nucleus surrounded by electrons that travel in a fixed circular path or orbits around the centre. |
| Postulates was given in the year/By | Niels Bohr in 1922. | Ernest Rutherford in 1913. |
| The experiment was based on | Hydrogen line spectrum. | Gold foil experiment. |
| Orbital size | This model also describes the relationship between the energy and size of the orbital, which says that "smallest orbital has the lowest energy". | Rutherford did not explain this relation of the orbital. |
| Energy levels | Bohr model was able to describe the discrete energy levels. | Rutherford's model did not describe discrete energy levels. |
| Electron frequencies | The emission of radiation by electrons is of specific frequencies. | The emission of radiation by electrons is of all frequencies. |
| Type of Emission Spectrum | The electron emission spectrum is the line spectrum. | The electron emission spectrum is a continuous spectrum. |
Definition of Bohr Theory
This theory was postulated by a Danish physicist named Neil Bohr in 1922 and has got its name as Bohr atomic model. This model is the modification of the Rutherford atomic model and so sometimes known as the ‘Rutherford-Bohr Model’.
Bohr’s model of the atom is rooted in quantum mechanics. Though it also has some pros and cons but considered as essential as this theory explained many points regarding atomic theory without any high-level maths. It also explained the spectral emission lines of atomic hydrogen known as the Rydberg formula.
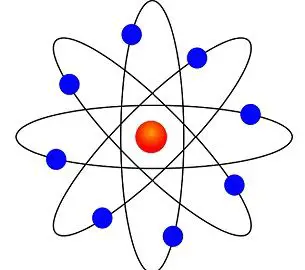
The Bohr Model is the planetary model which states that electrons move in a specified path known as an orbital shell. He also explained that this orbital shell has a fixed energy level. The Bohr theory is one step ahead of Rutherford’s model, where it described electrons and different energy levels. The Bohr model consists of small negatively charged electrons, which orbits or revolve around the positively charged nucleus.
Postulates of the Bohr Atomic Model
- Electrons move around the nucleus in orbits or shells or energy levels; it is the fixed circular path.
- The orbits are said to the “stationary orbits”.
- Each orbit has a certain amount of energy and size.
- The energy of the orbit corresponds to its size.
- The smallest orbit will have the lowest energy.
- Radiation is emitted or absorbed when electrons move from one to another orbit.
Drawbacks of the Bohr Model
- It does not follow the Heisenberg Uncertainty Principle.
- This model provides the incorrect value of the orbital angular momentum.
- It does not properly describe the spectra of larger atoms.
- It failed in explaining the Zeeman Effect.
Definition of Rutherford Theory
In 1897, J.J Thomson’s students put up the ‘Plum Pudding model‘ incorrectly and found atoms consist of subatomic particles called electrons and protons. Though he did not make it clear about the arrangement of such particles within the atom. Later on, in 1911, Ernest Rutherford, with his coworkers tested Thomson’s hypothesis by doing the ‘gold foil experiment’.
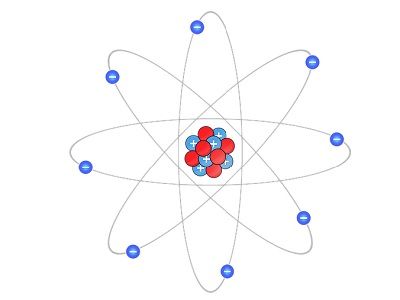
Rutherford took objection against the ‘plum pudding model’ and describe the planetary model. He described the planetary model, where the positively charged carried by an atom and its mass is present at its center or nucleus.
Rutherford with his coworkers Hans Geiger and Ernest Marsden performed historical experiments, that would change the concept of the model of an atom. In ‘The Gold Foil Experiment‘ they bombarded fast-moving alpha particles on the very thin sheets of gold foil. The alpha particles are the natural radioactive particles that are positively charged.
The experiment aimed to observe the deflection of alpha particles through the sheet, and for this, he put a screen of zinc sulfide around the gold foil.
Postulates of Rutherford Atomic Model based on the Gold Foil Experiment
- An atom contains positively charged particles. A significant portion of an atom is present in a small area called a nucleus of an atom.
- The nucleus of an atom is surrounded by negatively charged particles known as electrons.
- An atom is electrically neutral or has no net charge, because of the presence of positive charge (nucleus) and negative charge as well.
- The size of the nucleus is too small in comparison with the size of an atom.
Drawbacks of the Rutherford Atomic Model
- Rutherford’s model does not explain the electromagnetic theory.
- It also does not explain the stability of an atom and the lines of the spectrum.
- One of the major drawbacks was the description of the arrangement of the electrons.
Key Differences Between Bohr and Rutherford’s Atomic Theory
Given points are the critical one to understand the difference between two theories:
- Rutherford’s theory says that in the center of an atom is a small positively charged nucleus, which is surrounded by negatively charged electrons. The Bohr atomic theory depicts an atom as a positively charged, small nucleus surrounded by electrons that travel in a fixed circular path or orbits around the center.
- Postulates were given in the year Niels Bohr in 1922, Ernest Rutherford in 1913.
- Bohr’s atomic theory was based on the Hydrogen line spectrum, while Rutherford’s atomic theory was on Gold foil.
- Rutherford did not explain the relation of the orbital. Though the Bohr atomic model also describes the relationship between the energy and size of the orbital, which says that the “smallest orbital has the lowest energy“.
- Bohr’s model was able to describe the discrete energy levels, but Rutherford’s model did not explain these.
- In the Bohr model electron emission spectrum is the line spectrum, while in the Rutherford model, it is a continuous spectrum.
Conclusion
In this article, we studied the comparison chart between two atomic models, with their advantages and disadvantages. Although they both explained the concept of atomic structure with minute variations. Bohr model is said to be the modification of the Rutherford model. However, such models have contributed towards advanced models of atomic structure.

Leave a Reply