Compounds and mixtures are two important categories of matter. The mixtures, on one side, are formed by the physical mixing of one or more than one substances. Whereas the compounds are produced by chemical interaction at the molecular level.
There is a proper chemical bonding between two or more elements at the time of compound formation. But no such thing occurs in the case of the mixture, as there is no chemical reaction between the components.
The mixtures have no such decided ratio of their components. In contrast, elements of a compound combine in a fixed proportion. In case this decided ratio changes, the desired compound won’t generate.
There is no involvement of energy in the mixture formation. Contrastingly, the formation of a compound depends upon the give and take of energy, i.e., the energy changes.
We can easily separate the components of the mixture. But, the compounds are chemically bonded, so we cannot separate them through physical processes.
In this section, we will provide you with the primary differences between compounds and mixtures.
Content: Mixtures Vs Compounds
Comparison Chart
| Basis for Comparison | Mixtures | Compounds |
|---|---|---|
| Meaning | Mixtures are the impure substances, made up of two or more physically mixed substances. They can be homogeneous or heterogeneous by nature. | Compounds are the pure form, made up of two or more chemically mixed elements. These are generally homogeneous. |
| Examples | Alloys like brass; steel, oceanic water (salt and water), mixtures of gases, etc. | Compounds like Baking soda, Methane, Salt, etc. |
| Composition | The substances which are found in the mixtures are not in fixed quantity, that means their ratio varies. | But in the case of compounds, the elements are present in fixed quantity, that means their ratio is fixed. |
| Properties | The properties of the mixtures also vary (not fixed) as it depends on the type of substances and the quantity by which these are being mixed. | For the particular type of compound, the properties are fixed and do not vary, as the elements present in the compounds are fixed and are in the fixed ratio. |
| Formula | Mixtures do not have a certain formula. | Compounds have a specific formula, depending on the constituents present. |
| Separation | The substances of the mixtures are easy to separate by different physical methods like filtration, chromatography, evaporation. | The elements are not easy to separate and if done than it is by chemical methods. |
| Substances | No new substances are formed from the mixtures, due to the unchangeable properties of its constituents. | There is always formation of the new substances, due to the mixing of the chemical properties of the different constituents. |
| Melting/Boiling point | Mixtures do not have fixed melting or boiling point. | The compound once formed, have fixed melting and boiling point. |
| Heat change | There is no heat change, or involvement of energy is observed when mixtures are made. | There is a heat change, and energy is used or released during the formation of the compounds, as it is a chemical reaction. |
What are Mixtures?
Chemistry considers mixtures as impure substances. This is because a mixture is simply a combination or a blend of several components. Here, the constituents have no chemical interaction at a molecular level. They are just physically put together, and thus, we can easily separate them by performing simple separation techniques.
When you observe around, you will find mixtures everywhere. The air we breathe, the soil we walk on, and the blood flowing in our veins; are all typical examples of mixtures.
Definition of Mixtures
A substance formed by physically mixing two or more different materials in a random proportion without any molecular bonding is referred to as a mixture.
Properties of Mixtures
- The components of mixtures have no chemical bonding between their respective molecules.
- Thus, the constituting elements don’t lose their originality, i.e., the characteristic properties during mixture formation.
- In mixtures, neither new chemical bonds form nor old bonds break.
- The mixtures don’t have a decided melting or boiling point.
- There is no give or take of energy at the mixture formation.
- We can separate constituents of the mixtures through simple separation methods, including:
- Sieving
- Distillation
- Decantation
- Filtration
Types of Mixtures
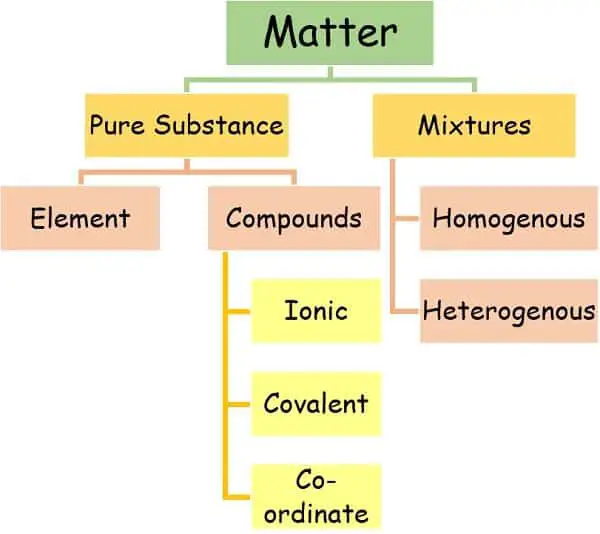
We can classify the mixtures into two prime categories: Homogenous and Heterogenous.
Homogenous Mixture
The homogenous mixtures are those which have a uniform distribution of their components. In other words, we can say that these mixtures have similar characteristic properties throughout the mixture.
For instance, when you mix sugar, salt and water together to form a solution, every drop of the solution will taste the same. This indicates that the particles are evenly homogenized within the solution.
Some more examples of homogenous mixtures are:
- Beverages: Like cola, fruit juice, tea, coffee etc.
- Air: Contains all its constituent gases uniformly distributed in a fixed proportion.
- Blood: All the components of blood, including RBCs, WBCs, platelets, antibodies etc., are evenly dispersed.
Properties of Homogenous Mixture
- In homogenous mixtures, the particles are so closely associated that their respective boundaries can’t be differentiated.
- The size of the particles in these sorts or mixtures is smaller than one nanometre.
- The particles of these mixtures do not exhibit the Tyndall effect.
- We can’t separate the mixture’s constituents particles through filtration, centrifugation or decantation.
Heterogeneous Mixtures
Heterogeneous mixtures are those mixtures that lack uniformity. Here, the components are randomly dispersed throughout the mixture. In most of heterogeneous mixtures, the distinction between the constituent particles is clearly visible.
For instance, if we mix sugar and rice together, the difference between both them is clearly visible. We can easily separate Rice and sugar particles either manually or by using a sieve.
Properties of Heterogeneous Mixtures
- The constituents of heterogeneous mixtures are readily identifiable as the boundaries of the particles are clearly separated.
- The size of the particles here is larger than one nanometre.
- The constituent particles of these mixtures exhibit the Tyndall effect.
- In these mixtures, the particles remain unevenly scattered.
- We can segregate the constituent particles of these mixtures by methods like decantation, filtration, sieving etc.
Example of Heterogenous MIxture
- Soil: The soil contains several components mixed together, such as sand, mud, gravel, pebbles, rocks, organic debris etc.
- Cereal and Milk: Our regular food items are mainly heterogeneous in nature. Milk and cereal are one such example.
What are Compounds?
Chemistry considers compounds as pure substances. A compound generates by the interaction of particles at their molecular level. The constituent particles of any compound are chemically bonded together.
As the components combine chemically, thus they lose their originality. That means the characteristic properties of a compound totally vary from its constituting elements.
Also, the components of a compound have a fixed ratio in which they interact. This infers that all the elements have their certain pre-decided proportion to form a specific compound.
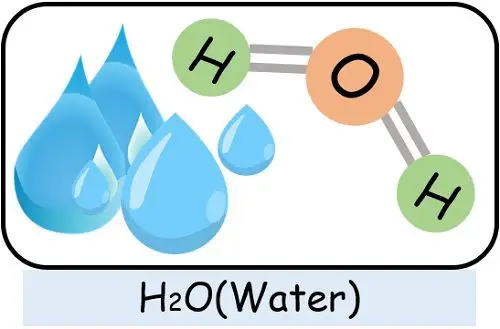
For instance, a water molecule can only form if 2 atoms of hydrogen and 1 atom of oxygen combine. In cases where the proportion of hydrogen or oxygen becomes more or less, it will generate something else but not water.
Definition of Compound
A pure substance formed by the chemical interaction between two or more elements combined in a definite ratio is referred to as a compound.
Properties of compounds
- The compounds have a fixed melting and boiling point.
- Because of the chemical bonding between the constituents, we can’t separate them by physical separation techniques.
- The properties of a compound and its components are different.
- The constituents combine in a fixed ratio to generate a compound.
- During the compound formation, old bonds break and new bonds form.
- There is the involvement of energy while the compound formation.
- We represent all the chemical compounds by specific chemical formulas.
Some common examples of Compounds
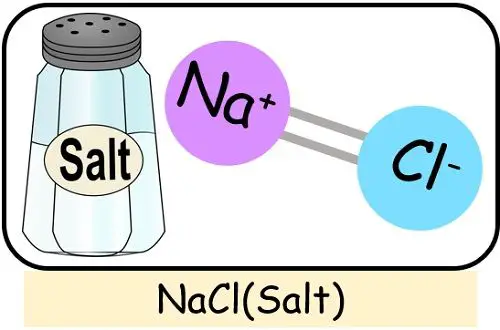
- Salt: Salt is a compound formed by the chemical reaction between sodium and chlorine.
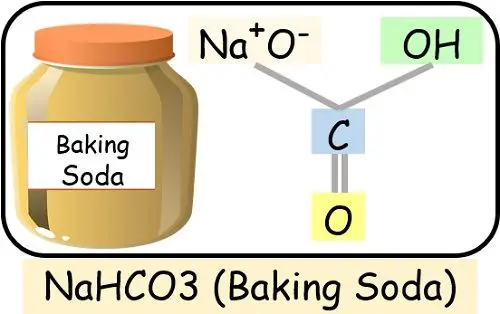
- Baking Soda: Composed of sodium, hydrogen, carbon and oxygen chemically interact together.
- Water: It contains two hydrogens and one oxygen covalently bound together.
- Sugar: It is an organic compound containing carbon, hydrogen and oxygen.
Types of Compound
We can categorize the compounds based on two major criteria:
1. Based on the type of bond
2. Based on the presence of carbon
1. Based on the type of bond
We can classify the compounds into three main types based on the bond existing between them.
Ionic Compounds
These compounds have an ionic bond between the constituting elements. This type of bond forms by the give and take of the electron between two atoms. In other words, the positive cation reacts with negative anions to produce the ionic bond.
Examples of such compounds are NaCl, H2O, MgCl2, CuSO4 etc.
Covalent Compounds
They possess covalent interaction between their components. The covalent bond generates due to the sharing of electrons between two atoms. Here, instead of completely giving or taking the electron, adjacent atoms mutually share the electrons.
Common examples of covalent compounds are CH4, NH3, H2, O2 etc.
Coordinate Compounds
These are neutral compounds that consist of a central metal ion. This coordinate centre remains surrounded array of ligands.
Examples of these compounds are [Co (NH3)6] Cl3, Cu2[Fe (CN)6] etc.
2. Based on the presence of carbon
On this basis, compounds are of two types:
Organic Compounds
The compounds containing carbon are organic compounds.
For example, CH4, NaCOOH, C2HCl3O, C2H2O2, HC6H7O7 etc.
Inorganic Compounds
The compounds deprive of carbon are inorganic.
For example, H2SO4, HCl, CaCl2, Na2SO4 etc.
Key Differences Between Mixtures and Compounds
- Mixtures are impure substances formed due to the physical mixing of two or more components. On the other side, compounds are pure substances. They form by the chemical interaction between the constituents at the molecular level.
- No chemical bonds form in the case of mixtures. While the chemical bonds form the compounds.
- The components of the mixture are not definite, but the compounds are in a fixed ratio.
- Separation of the components of the mixture is easily possible by the physical separation methods. In contrast, the elements linked by chemical bonding in the compounds can’t be separated by these methods.
- There is no energy involvement while mixture formation. But there is a give and take of energy during the compound formation.
- Examples of mixtures are Alloys like brass, bismuth, chromium, oceanic water (salt and water), etc.
While Sodium Chloride, Baking soda, Methane, Salt, etc., are examples of the compounds.
Conclusion
You can easily distinguish the properties of mixtures and compounds with this article. This difference can be helpful in understanding these basic topics of chemistry. As well as it will provide general information about the things in your household life.

H says
Very helpful
Kritika Garg says
Thanks a lot it helped me and the data is give in tabular form which makes it interesting and attractive. Thank you!
Sarah says
Wow very informative it really helped me so that is a thumbs up 👍👍👍
Stan says
Extremely helpful with revision for my upcoming science test! I reccomend to anyone who is stuck!